
The first oral contraceptive for men
Now in Phase 2 clinical trials.
Explore the science
See our studies
YCT‑529 is an investigational agent. We do not claim that it is safe or effective for any use.
Why we are working on this
Women have had the Pill for over 60 years. Men still have two options: condoms or vasectomy.
Over 60% of men would try a new contraceptive option.
A daily pill. Non-hormonal. In clinical studies.
Biology we are studying
YCT-529 is designed to block retinoic acid receptor-alpha (RAR-alpha), a receptor essential for sperm production. In preclinical studies, the effect on sperm production reversed after stopping treatment.
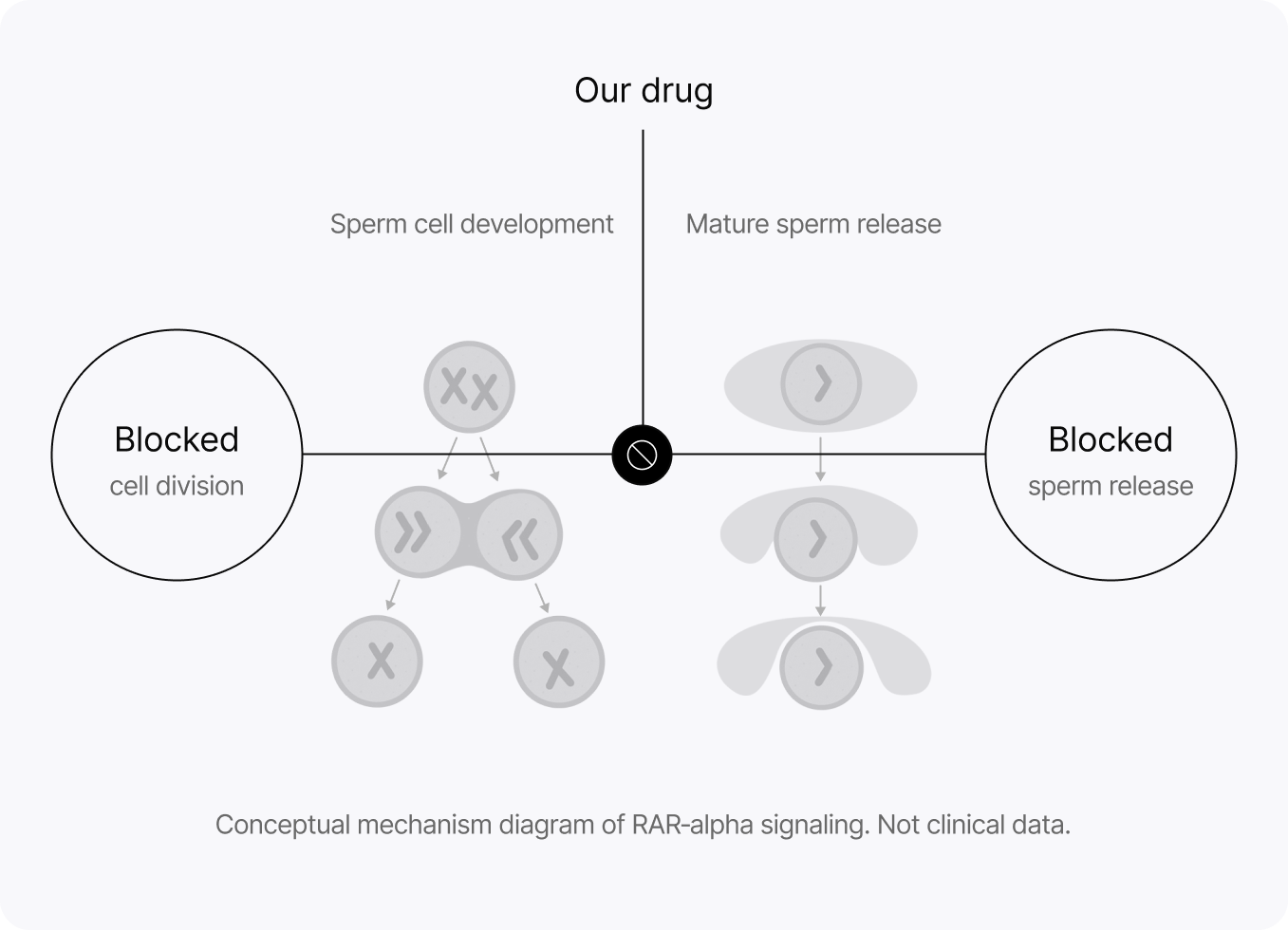
Clinical development
YCT-529 — oral, non-hormonal male contraception (investigational)
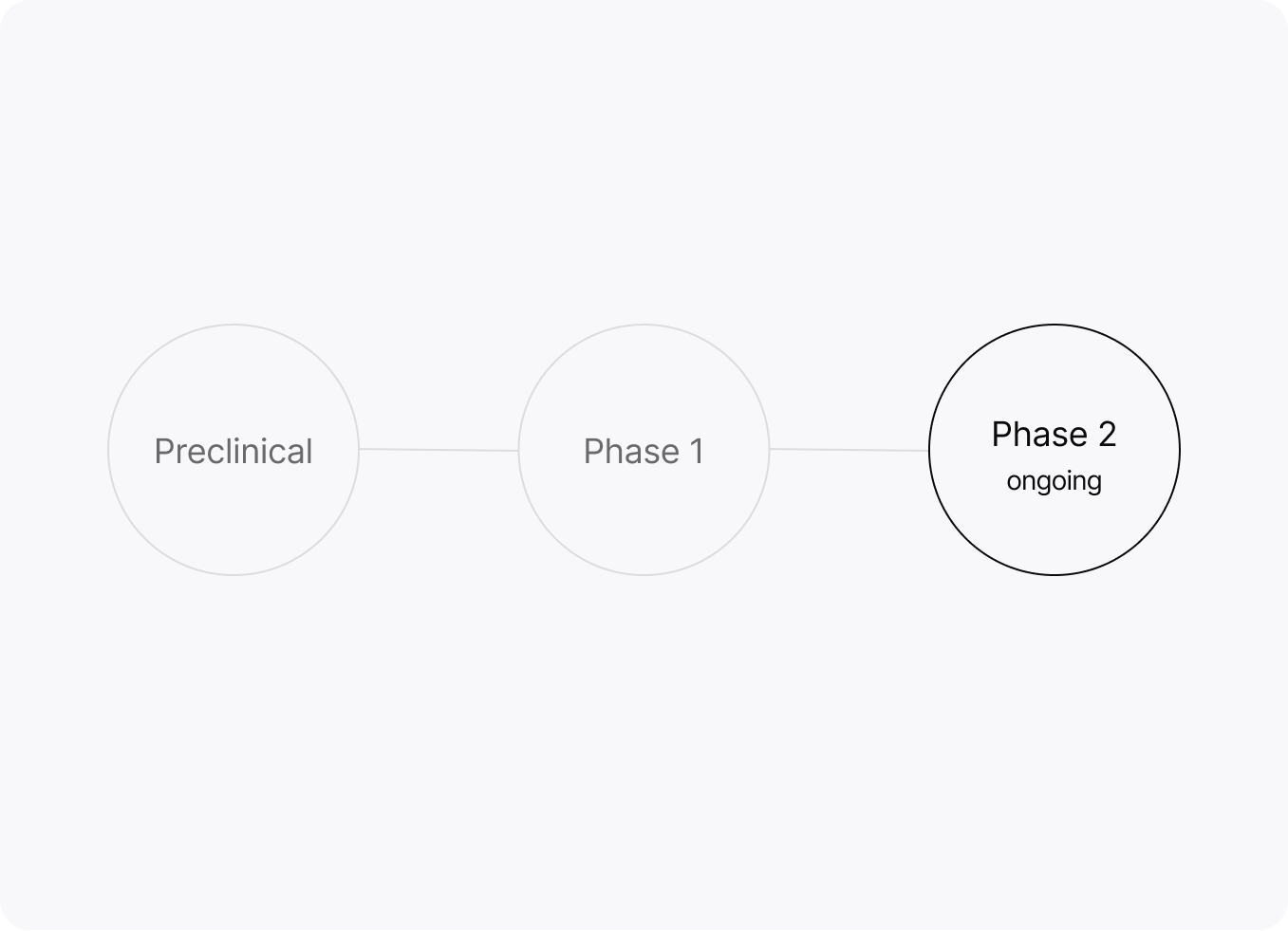
We sponsor and collaborate on clinical studies to evaluate YCT-529. Current focus areas include safety, tolerability, and pharmacokinetics.
View on ClinicalTrials.gov
Contact:
clinicaltrials@yourchoicetx.com
Publications and presentations
We share our research through peer‑reviewed publications and scientific meetings
Founders

Akash Bakshi, MSc
Co‑founder & CEO
Nadja Mannowetz, PhD
Co‑founder & CSO
Open roles
Newsroom
Company updates and selected media coverage
Press releases
About YourChoice Therapeutics
YourChoice Therapeutics is developing hormone‑free, oral investigational male contraception. YCT‑529 is a selective retinoic acid receptor‑alpha (RAR‑alpha) antagonist under clinical investigation and has not been approved by any regulatory authority.
Investor access
Request access to our investor materials. Investor information is shared at our discretion and may require a confidentiality agreement.
Request investor access
© 2026 YourChoice Therapeutics.
All rights reserved.

The first oral contraceptive for men
Now in Phase 2 clinical trials.
YCT‑529 is an investigational agent. We do not claim that it is safe or effective for any use.
Why we are working on this

Women have had the Pill for over 60 years. Men still have two options: condoms or vasectomy.

Over 60% of men would try a new contraceptive option.

A daily pill. Non-hormonal. In clinical studies.
Biology we are studying
YCT-529 is designed to block retinoic acid receptor-alpha (RAR-alpha), a receptor essential for sperm production. In preclinical studies, the effect on sperm production reversed after stopping treatment.

Clinical development
YCT-529 — oral, non-hormonal male contraception (investigational)

We sponsor and collaborate on clinical studies to evaluate YCT-529. Current focus areas include safety, tolerability, and pharmacokinetics.
View on ClinicalTrials.gov
Contact:
clinicaltrials@yourchoicetx.com
Publications and presentations
We share our research through peer‑reviewed publications and scientific meetings
Founders

Akash Bakshi, MSc
Co‑founder & CEO
Nadja Mannowetz, PhD
Co‑founder & CSO
Open roles
Newsroom
Company updates and selected media coverage
Bloomberg Businessweek - The Future of Male Birth Control Could Be Pills, Gels and Implants - January 28, 2026
Press releases
About YourChoice Therapeutics
YourChoice Therapeutics is developing hormone‑free, oral investigational male contraception. YCT‑529 is a selective retinoic acid receptor‑alpha (RAR‑alpha) antagonist under clinical investigation and has not been approved by any regulatory authority.
Investor access
Request access to our investor materials. Investor information is shared at our discretion and may require a confidentiality agreement.
Request investor access
© 2026 YourChoice Therapeutics. All rights reserved.

The first oral contraceptive for men
Now in Phase 2 clinical trials.
YCT‑529 is an investigational agent. We do not claim that it is safe or effective for any use.
Why we are working on this

Women have had the Pill for over 60 years. Men still have two options: condoms or vasectomy.

Over 60% of men would try a new contraceptive option.

A daily pill. Non-hormonal. In clinical studies.
Biology we are studying
YCT-529 is designed to block retinoic acid receptor-alpha (RAR-alpha), a receptor essential for sperm production. In preclinical studies, the effect on sperm production reversed after stopping treatment.


Clinical development
YCT-529 — oral, non-hormonal male contraception (investigational)
We sponsor and collaborate on clinical studies to evaluate YCT-529. Current focus areas include safety, tolerability, and pharmacokinetics.
View on ClinicalTrials.gov
Contact:
clinicaltrials@yourchoicetx.com
Publications and presentations
We share our research through peer‑reviewed publications and scientific meetings
Founders

Akash Bakshi, MSc
Co‑founder & CEO
Nadja Mannowetz, PhD
Co‑founder & CSO
Open roles
Newsroom
Company updates and selected media coverage
Press releases
About YourChoice Therapeutics
YourChoice Therapeutics is developing hormone‑free, oral investigational male contraception. YCT‑529 is a selective retinoic acid receptor‑alpha (RAR‑alpha) antagonist under clinical investigation and has not been approved by any regulatory authority.
Investor access
Request access to our investor materials. Investor information is shared at our discretion and may require a confidentiality agreement.
Request investor access
© 2026 YourChoice Therapeutics. All rights reserved.

The first oral contraceptive for men
Now in Phase 2 clinical trials.
YCT‑529 is an investigational agent. We do not claim that it is safe or effective for any use.
Why we are working on this

Women have had the Pill for over 60 years. Men still have two options: condoms or vasectomy.

Over 60% of men would try a new contraceptive option.

A daily pill. Non-hormonal. In clinical studies.
Biology we are studying
YCT-529 is designed to block retinoic acid receptor-alpha (RAR-alpha), a receptor essential for sperm production. In preclinical studies, the effect on sperm production reversed after stopping treatment.


Clinical development
YCT-529 — oral, non-hormonal male contraception (investigational)
We sponsor and collaborate on clinical studies to evaluate YCT-529. Current focus areas include safety, tolerability, and pharmacokinetics.
View on ClinicalTrials.gov
Contact:
clinicaltrials@yourchoicetx.com
Publications and presentations
We share our research through peer‑reviewed publications and scientific meetings
Founders

Akash Bakshi, MSc
Co‑founder & CEO
Nadja Mannowetz, PhD
Co‑founder & CSO
Open roles
Newsroom
Company updates and selected media coverage
Press releases
About YourChoice Therapeutics
YourChoice Therapeutics is developing hormone‑free, oral investigational male contraception. YCT‑529 is a selective retinoic acid receptor‑alpha (RAR‑alpha) antagonist under clinical investigation and has not been approved by any regulatory authority.
Investor access
Request access to our investor materials. Investor information is shared at our discretion and may require a confidentiality agreement.
Request investor access
© 2026 YourChoice Therapeutics. All rights reserved.

The first oral contraceptive for men
Now in Phase 2 clinical trials.
YCT‑529 is an investigational agent. We do not claim that it is safe or effective for any use.
Why we are working on this

Women have had the Pill for over 60 years. Men still have two options: condoms or vasectomy.

Over 60% of men would try a new contraceptive option.

A daily pill. Non-hormonal. In clinical studies.
Biology we are studying
YCT-529 is designed to block retinoic acid receptor-alpha (RAR-alpha), a receptor essential for sperm production. In preclinical studies, the effect on sperm production reversed after stopping treatment.


Clinical development
YCT-529 — oral, non-hormonal male contraception (investigational)
We sponsor and collaborate on clinical studies to evaluate YCT-529. Current focus areas include safety, tolerability, and pharmacokinetics.
View on ClinicalTrials.gov
Contact:
clinicaltrials@yourchoicetx.com
Publications and presentations
We share our research through peer‑reviewed publications and scientific meetings
Founders

Akash Bakshi, MSc
Co‑founder & CEO
Nadja Mannowetz, PhD
Co‑founder & CSO
Open roles
Newsroom
Company updates and selected media coverage
Live Science - Male birth control pill passes early safety test, with more trials underway - July 2025
Press releases
About YourChoice Therapeutics
YourChoice Therapeutics is developing hormone‑free, oral investigational male contraception. YCT‑529 is a selective retinoic acid receptor‑alpha (RAR‑alpha) antagonist under clinical investigation and has not been approved by any regulatory authority.
Investor access
Request access to our investor materials. Investor information is shared at our discretion and may require a confidentiality agreement.
Request investor access
© 2026 YourChoice Therapeutics. All rights reserved.
Media inquiries: press@yourchoicetx.com
Media inquiries: press@yourchoicetx.com
General inquiries: info@yourchoicetx.com
Terms
Privacy



